The HU CFAR Clinical Core connects HIV-related research cohorts with resources. In turn, many of these studies have data and specimens available for further investigation. If your research can benefit from access to data and specimens from these Cohorts, please reach out to the respective study contact. Conversely, if you have overseen an HIV Cohort and have data and specimens that you are willing to share and want the study to be listed in the HU CFAR Clinical Cohort Catalogue, please submit the form directly via the Cohort Submission Form.
The Clinical Core provides key infrastructure for a number of studies led by HU CFAR investigators. Clinical research staff are involved in investigator-initiated HIV clinical trials with the ability to assist HU CFAR investigators. Please contact the Clinical Core Liaison for any questions related to accessing these services at the following locations:
- Massachusetts General Hospital (MGH)
- Brigham and Women’s Hospital (BWH)
- Beth Israel Deaconess Medical Center (BIDMC)
- Lemuel Shattuck Hospital (LSH)
- Fenway Health (FH)
- Boston Children’s Hospital (BHC)
Botswana Prospective Cancer Cohort
- Principal Investigator(s): Scott Dryden-Peterson
- Study Contact: Scott Dryden-Peterson, +2.677.447.2522, scott_peterson@post.harvard.edu
- Description of Subjects: Botswana adults with a new diagnosis of cancer
- Location (Domestic vs. International): Botswana Harvard AIDS Institute
- Enrollment Period: 2010-ongoing
- Enrollment Goal: 3000
- Key Exclusion and Inclusion Criteria: cancer diagnosis, both HIV infected and HIV uninfected
- Available Biological Specimens: N/A
- How to Request Access: email study PI
- Available Data Description: longitudinal outcome, baseline staging (CD4, VL)
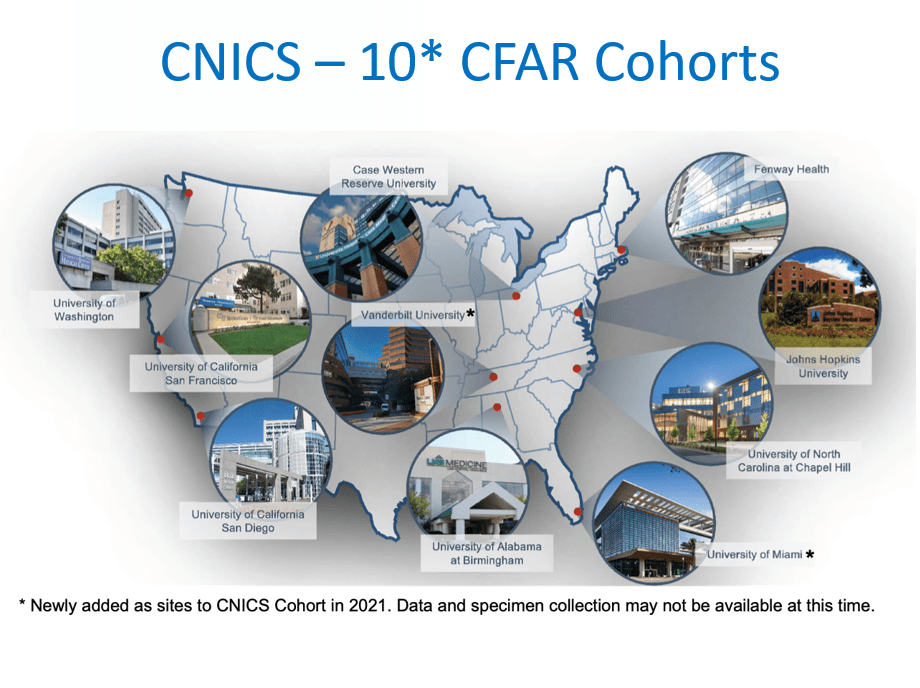
CFAR Network of Integrated Clinical Systems (C-NICS)
- Principal Investigator(s): Ken Mayer, MD
- Study Contact:, Ken Mayer, Tel: 617-927-6087, kmayer@fenwayhealth.org
- Description of Subjects: 30,000 PLHIV in care at 8 HIV primary care centers
- Location (Domestic vs. International): Fenway Health; UNC; UAB; Case Wester; UCSD; UCSF; U Wash
- Enrollment Period: 2005-ongoing
- Enrollment Goal: open cohort, >30,000 PLHIV.
- Key Exclusion and Inclusion Criteria: HIV-infected and in care at one of the participating sites is required to be part of CNICS, but Fenway has had an EHR since 1997, so case-control studies with uninfected patients are also feasible
- Available Biological Specimens and Data: Yes, Blood, serum, or plasma, Peripheral Blood Mononuclear Cells, Other (Cell Pellets)
- How to Request Access: email study contact directly at cgrasso@fenwayhealth.org
- Link to Cohort Website: http://www.uab.edu/cnics/
- Available Data Description: Demographics, risk factor, death data and longitudinal lab values (including CD4 and viral load), diagnosis, medication, encounters, and patient reported outcomes.
- Other Information: The CFAR Network of Integrated Clinical Systems (CNICS) research network, is a NIH sponsored study, which is the first electronic medical records-based resource network poised to integrate clinical data from the large and diverse population of HIV-infected persons. CNICS supports HIV clinical outcomes and comparative effectiveness research using data collected from patients receiving care at one of 8 US-funded Center for AIDS Research (CFAR) sites. Fenway Health is one of the eight sites and has participated in this study since 2001. CNICS directly reflects the outcomes of clinical decisions and management options made daily in the care of HIV infected individuals and is helping to evaluate HIV treatment interventions under the real world conditions of a primary care practice.
Control of HIV after Antiretroviral Medication Pause (CHAMP)
- Principal Investigator(s): Jonathan Li
- Study Contact: Jonathan Li, jli@bwh.harvard.edu
- Description of Subjects: Post-treatment controllers
- Location (Domestic vs. International): United States, Canada
- Enrollment Period: n/a
- Enrollment Goal: 200
- Key Exclusion and Inclusion Criteria: Discontinued antiretroviral therapy with viral loads <=400 HIV-1 RNA copies/mL at 2/3 of time points for at least 24 weeks
- Available Biological Specimens:
- Blood, serum, or plasma
- DNA
- Peripheral Blood Mononuclear Cells
- How to Request Access: Email study contact
- Available Data Description: Clinical and immunological data
- Optional Additional Comments: If “Elite” is removed from the “Elite Controllers” Keyword, then it would apply to this study.
Community Health Applied Research Network (CHARN)
- Principal Investigator(s): Kenneth Mayer, MD
- Study Contact: Kenneth Mayer, MD
- Description of Subjects: Patients receiving primary care at participating community health centers across the U.S.
- Location (Domestic vs. International): Boston, MA; greater MA; New England
- Enrollment Period: 2007-2014
- Enrollment Goal: >50,000
- Key Exclusion and Inclusion Criteria: Patients with two or more visits at a participating community health center since 2007
- Available Biological Specimens: N/A
- How to Request Access: Email study contact person directly
- Available Data Description: longitudonal data, labs, medications, demographics, diagnosis, insurance status, encounters
- Optional Additional Comments: CHARN is a unique network of community health centers and universities that was established to conduct patient-centered outcome research among underserved populations. It represents the first time that such a large and diverse group of community health centers has come together in this context. The Fenway node includes Fenway Health, Boston MA; Chase Brexton Health Services, Baltimore MD; Beaufort Jasper Hampton Comprehensive Health Services, Ridgeland SC. CHARN is comprised for 4 nodes: Fenway Health, AAPCHO, OCHIN, and Alliance of Chicago which represent 17 CHCs across the U.S. Each node has an academic partner: University of Washington (Fenway); Oregon Health Sciences University (OCHIN); Northwestern (Alliance); and UCLA (AAPCHO). CHARN is coordinated by the Kaiser Permanente Center for Health Research in Portland, OR.
Cotrimoxazole safety study
- Principal Investigator(s): Scott Dryden-Peterson
- Study Contact: Scott Dryden-Peterson, +2.677.447.2522, scott_peterson@post.harvard.edu
- Description of Subjects: Infants born to HIV-infected women
- Location (Domestic vs. International): Botswana
- Enrollment Period: 2008-2009
- Enrollment Goal: 444 mother-infant pairs
- Key Exclusion and Inclusion Criteria: Inclusion: live born infants from HIV-infected mothers in Botswana
- Available Biological Specimens:
- Blood, serum, or plasma
- How to Request Access: Email PI
- Available Data Description: longitudinal outcome data, maternal health history
- Optional Additional Comments: biologic specimens only available for infants born to HIV-infected mothers. No maternal specimens available. Few infants HIV-infected.
Epidemiology of Cardiovascular Disease among People Living with HIV in Rural Uganda
- Principal Investigator(s): Mark Siedner
- Study Contact: Mark Siedner, 617-726-4686, msiedner@partners.org
- Description of Subjects: People Living with HIV and Age and Gender-matched Controls in Southwestern Uganda
- Location (Domestic vs. International): Mbarara University of Science and Technology, Mbarara, Uganda
- Enrollment Period: 2013-2016
- Enrollment Goal: 500
- Key Inclusion Criteria: Age greater than 40, living within catchment area of Mbarara Regional Referral Hospital, Uganda
- Available Biological Specimens:
- Blood, serum, or plasma
- Tissues and Stool
- How to Request Access: Contact PI
- Available Data Description: Metabolic Assays, Stool Microbiome, Inflammatory Markers, Cardiovascular Disease Surrogates (EKG, ABI, Carotid U/S)
Fenway Cohort affiliated with (North American- AIDS Cohort Collaboration on Research and Design (NA-ACCORD))
- Principal Investigator(s): Kenneth Mayer, MD
- Study Contact: Chris Grasso, MPH, 617-927-6018, cgrasso@fenwayhealth.org
- Description of Subjects: Fenway Health medical patients who have had at least 2 HIV primary care visits at Fenway in a 12 month period and are 18 years or older
- Location (Domestic vs. International): Fenway has participants from all over the New England geographic area. Fenway’s facilities are located throughout Boston, MA.
- Enrollment Period: 1997 to present (ongoing)
- Enrollment Goal: Presently, Fenway has over 2,600 participants. Enrollment is ongoing.
- Key Exclusion and Inclusion Criteria: Inclusion criteria: HIV-infected persons who are 18 years or older and have had at least 2 HIV primary care visits at Fenway in a 12 month period; and vital statistics, gender, year of birth, and first visit must be known . Exclusions: transgender, tran
- Available Biological Specimens:
- Blood, serum, or plasma
- Peripheral Blood Mononuclear Cells
- Other (Cell Pellets)
- How to Request Access: Email study contact person directly
- Available Data Description: Demographics, risk factor, death data and longitudinal lab values (including CD4 and viral load), diagnosis, medication, and encounters
- Optional Additional Comments: Fenway was accepted into the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) database research project in 2011. NA-ACCORD is part of the International epidemiologic Databases to Evaluate AIDS (IeDEA) which began in 2006. Comprised of 25 collaborating cohorts, NA-ACCORD is designed to be widely representative of HIV care in the United States and Canada. NA-ACCORD, with Johns Hopkins University as the lead, is one of seven regional HIV collaborations supported by the NIH as part of the International Epidemiologic Databases to Evaluate of AIDS (IeDEA).
FLOURISH Study (Following Longitudinal Outcomes to Understand, Report, Intervene and Sustain Health for Infants, Children, Adolescent who are HIV Exposed Uninfected) – R33HD103099
- Principal Investigator(s): Dr. Kathleen Powis (MGH), Dr. Jennifer Jao (Lurie Children’s), Dr. Joseph Makhema (BHP)
- Study Contact: Kathleen Powis, Associate Professor, Massachusetts General Hospital, 617-947-9150, kpowis@mgh.harvard.edu
- Description of Subjects: Botswana maternal-child pairs, with children who are HIV-exposed and uninfected and HIV-unexposed and uninfected
- Location (Domestic vs. International): Botswana Harvard Health Partnership (BHP)
- Enrollment Period: 2021-ongoing
- Enrollment Goal: 2,075 maternal-child pairs
- Key Exclusion and Inclusion Criteria:
- Exclusion criteria: Child living with HIV
- Available Biological Specimens: Yes
- How to Request Access: Contact Dr. Kathleen Powis (kpowis@mgh.harvard.edu)
- Available Data Description:
- Longitudinal maternal and child clinical, sociodemographic, and laboratory data;
- In-person baseline
- Quarterly follow-up phone calls
- In-person follow-up
- Longitudinal maternal and child clinical, sociodemographic, and laboratory data;
Health Outcomes around Pregnancy and Exposure to HIV/ARVs (HOPE – multicenter 12 sites)
- Principal Investigator(s): Dr. Deborah Kacanek & Dr. Paige Williams (HSPH), Dr. Kathleen Powis (MGH), Dr. Ellen Chadwick (Northwestern)
- Study Contact: Dr. Deborah Kacanek, Senior Research Scientist, 617-432-2833, dkacanek@sdac.harvard.edu and Dr. Kathleen Powis, Associate Professor, Massachusetts General Hospital, 617-643-2054, kpowis@mgh.harvard.edu
- Description of Subjects: Women of reproductive age (>18 and <40 years of age) living with HIV
- Location (Domestic vs. International): 12 clinical sites across the U.S., including Puerto Rico (see map of clinical sites)
- Enrollment Period: 2022-present
- Enrollment Goal: 1630 participants; enrollment is open and ongoing
- Key Exclusion and Inclusion Criteria:
- Inclusion criteria – Female based on biological sex assignment at birth; HIV infection as documented in the medical record; if pregnant or parous, 18 to < 40 years of age; if nulliparous and non-pregnant, 18 to ≤30 years of age; if pregnant, gestational age ≥13 weeks by best obstetric estimates; willingness to provide access to existing medical records; willingness to participate and provide legal consent/assent; and able to complete study assessments in English or Spanish.
- Exclusion criteria – Individuals who meet any of the criteria listed below are not eligible for enrollment: currently incarcerated; age ≥ 40 years or <18; male based on biological sex assignment at birth (even if self-identified as female); any concurrent participation in other studies not approved by the HOPE protocol team.
- Available Biological Specimens:
- Blood, serum, or plasma
- DNA
- Peripheral Blood Mononuclear Cells
- Saliva
- Hair
- How to Request Access: See https://phacsstudy.org/Our-Research/HOPE
- Available Data Description: All of the above, as well as extensive ND assessments
HIV and Aging Cohort Study
- Principal Investigator(s): Nina Lin
- Study Contact: Nina Lin, nhlin@bu.edu
- Description of Subjects: HIV+ on ART who are </= 35 yo or >/=50yo with no hepatitis co-infection
- Location (Domestic vs. International): Domestic, BWH, BIDMC, MGH
- Enrollment Period: 2011-present
- Enrollment Goal:
- Key Exclusion and Inclusion Criteria: Started on combination ART, virally suppressed for >/=6 months, no co-infection with hepatitis B/C
- Available Biological Specimens and Data: Peripheral Blood Mononuclear Cells, Plasma
- How to Request Access: Contact Dr. Nina Lin
- Available Data Description: CD4, VL, PMH
HIV Controllers
- Principal Investigator(s): Nikolaus Jilg
- Study Contact: Clinical Research Team, 857-268-7257, ragonclinicalresearch@mgh.harvard.edu
- Description of Subjects: HIV positive, HIV-RNA <2000 cop/ml, off cART
- Location (Domestic vs. International): Domestic
- Enrollment Period: 2005 – ongoing
- Current Accrued Enrollment: 1400 participants since 2010 maintaining a viral load below 2,000 copies/mL in absence of antiretroviral therapy
- Enrollment Goal: 1000
- Key Exclusion and Inclusion Criteria: Inclusion HIV-RNA < 2000 copies x 3 determinations over > 12 months. Exclusion current cART
- Available Biological Specimens and Data: Yes, plasma, DNA, PBMC and B cell lines
- How to Request Access: ragonclinicalresearch@mgh.harvard.edu
- Mechanism for Requesting Samples: ragonclinicalresearch@mgh.harvard.edu
- Data Description: In 2010, primary and secondary analysis was completed of the genome wide association study and published in Science and Human Molecular Genetics.
HIV Chronic Infection
- Principal Investigator(s): Bruce Walker
- Study Contact: Clinical Research Team, 857-268-7257, ragonclinicalresearch@mgh.harvard.edu
- Description of Subjects: HIV positive, treated or untreated
- Location (Domestic vs. International): Domestic
- Enrollment Period: 1999-ongoing
- Enrollment Goal:
- Key Exclusion and Inclusion Criteria: Inclusion: 18-75 yo, HIV positive
- Available Biological Specimens and Data: plasma, DNA, PBMC and B cell lines
HIV-associated Seizures and Epilepsy in Zambia
- Principal Investigator(s): Omar K. Siddiqi
- Study Contact: Omar K. Siddiqi, +260 979365956, osiddiqi@bidmc.harvard.edu
- Description of Subjects: HIV+ adults with new onset seizure
- Location (Domestic vs. International): Lusaka, Zambia – University of Zambia School of Medicine
- Enrollment Period: 2011 – 2013
- Enrollment Goal: 100
- Key Exclusion and Inclusion Criteria: > 18 yo, new onset seizure in last 2 weeks, 1st seizure as an adult, no prior diagnosis of epilepsy.
- Available Biological Specimens: Other (CSF) cerebrospinal fluid
- How to Request Access: email study contact directly osiddiqi@bidmc.harvard.edu
- Available Data Description: CD4, EEG, CSF DNA PCR, CSF CrAg, mortality, seizure recurrence, neuroimaging.
HIV Research Network (HIVRN)
- Principal Investigator(s): Ken Mayer
- Study Contact: Ken Mayer, DrPH, 617 927 6087, kmayer@fenwayhealth.org
- Description of Subjects: HIV-infected patients aged 18 and older receiving medical care at Fenway Health. (HIVRN includes pediatric data, but Fenway Health only submits adult patient data)
- HIV+
- HIV/HCV Co-infected
- Location (Domestic vs. International): Fenway has participants from all over New England
- Enrollment Period: 2001-present
- Enrollment Goal: Presently, Fenway has over 3,000 participants. Enrollment is ongoing.
- Key Exclusion and Inclusion Criteria: Inclusion criteria: HIV-infected, at least 1 in-person medical visit, and at least 1 CD4 count in the observation period. Fenway only includes patients 18yrs and older.
- Available Biological Specimens: Blood, serum, or plasma, Peripheral Blood Mononuclear Cells
- How to Request Access: Email the Fenway study coordinator
- Available Data Description: Longitudinal laboratory data (including CD4 counts, viral loads), demographics, risk factors, vitals, phenotypes, vaccinations, primary care procedures, Diagnoses (ADI, MH, SA, Co-morbidities), medications, limited inpatient, outpatient, and ER visits
- Optional Additional Comments: Fenway is providing data to HIVRN, a national consortium of HIV care providers, whose goal is to gather and analyze data on accessibility, quality, utilization, safety, and costs of health care services provided to persons with HIV disease to inform health policy and for research. Fenway submits data annually on our HIV-infected patients. Data submitted covers a broad range of topics including, but not limited to: demographics, ARV history, ART and current medications, OI prophylaxis, infectious disease labs, vaccinations, AIDS defining illnesses, mental health and substance abuse diagnoses, and inpatient and outpatient visits. HIVRN is sponsored by AHRQ and Johns Hopkins University School of Medicine is the lead site.
HIV Tissues
- Principal Investigator(s): Douglas Kwon
- Study Contact: Douglas Kwon, (857) 268-7009, dkwon@partners.org
- Description of Subjects: HIV positive and negative
- Location (Domestic vs. International): Domestic
- Enrollment Period: 2010-ongoing
- Enrollment Goal: 200
- Key Exclusion and Inclusion Criteria: Inclusion: 18-75 yo, Exclusion: IBD, HCV
- Available Biological Specimens and Data: formalin fixed gut tissue, stool
HIV Reservoir Dynamics During Antiretroviral Therapy (The HIV Eradication and Latency [HEAL] Cohort)
- Principal Investigator(s): Athe Tsibris
- Study Contact:, Athe Tsibris, atsibris@bwh.harvard.edu
- Description of Subjects: Virologically suppressed participants with HIV
- Location (Domestic vs. International): Boston, MA (BWH, MGH, BMC)
- Enrollment Period: 2015
- Enrollment Goal: 200
- Key Exclusion and Inclusion Criteria: HIV infection, must be taking (or expected to begin taking) a combination antiretroviral therapy regimen, hemoglobin ≥ 8.0 g/dL. Excludes need for systemic chemotherapy, bone marrow transplant, pregnancy, and need for immunosuppressive therapy
- Available Biological Specimens and Data: Yes, Blood, serum, or plasma, Peripheral Blood Mononuclear Cells, Other (leukapheresis samples)
- How to Request Access: email HEAL PI directly
- Available Data Description: CD4 counts, virus loads, treatment history, duration of therapy, duration of virologic suppression.
- Other Information: Gut and skin biopsies will be added in future
Kaposi’s sarcoma treatment in Kenya and Nigeria
- Principal Investigator(s): Esther Freeman
- Study Contact: efreeman@mgh.harvard.edu
- Description of Subjects: Adults with HIV-related Kaposi’s sarcoma in Kenya and Nigeria
- Location (Domestic vs. International): AMPATH (Eldoret, Kenya), Institute of Human Virology (Abuja, Nigeria)
- Enrollment Period: 2009-2012
- Enrollment Goal: All KS patients diagnosed at these sites during enrollment period
- Key Exclusion and Inclusion Criteria: Diagnosed with KS between 2009-2012
- Available Biological Specimens and Data: Other (Skin biopsy)
- How to Request Access: please email study contact directly
- Available Data Description: CD4
- Other Information: Cohort through IeDEA network
Linkage & Retention: A Randomized Trial to Optimize HIV/TB care in South Africa
- Principal Investigator(s): Ingrid V. Bassett, MD, MPH
- Study Contact: Ingrid V. Bassett, MD, MPH, 617 726 0637, ibassett@partners.org
- Description of Subjects: Undergoing HIV testing at 4 outpatient sites in Durban, South Africa
- Location (Domestic vs. International): Durban, South Africa
- Enrollment Period: 2010-2013
- Enrollment Goal: 4903 total (1899 HIV infected, 3,004 HIV uninfected)
- Key Exclusion and Inclusion Criteria: Adults, presenting for outpatient care, voluntarily undergoing HIV testing
- Available Biological Specimens: Other (Stored sputum specimens)
- How to Request Access: email study contact directly ibassett@partners.org
- Available Data Description: AFB smear, TB culture with drug susceptibility testing, baseline CD4
- Optional Additional Comments: 9 month follow up data available, including linkage and retention in HIV and TB care, also 5 year mortality data using crossmatching with National Population Registry
Novel Strategies for Monitoring Transmitted Drug Resistance in Botswana
- Principal Investigator(s): Christopher Rowley
- Study Contact: Christopher Rowley, 617 432-3549, crowley1@bidmc.harvar.edu
- Description of Subjects: pregnant females, 18-25, and adults (male/female) 18-60
- Location (Domestic vs. International): Botswana
- Enrollment Period: 2012-2015
- Enrollment Goal: 550 participants
- Key Exclusion and Inclusion Criteria: Treatment-naive
- Available Biological Specimens:
- Blood, serum, or plasma
- Other (buffy coat)
- How to Request Access: email study contact
- Available Data Description: CD4 counts, viral loads, age, sex
The MATCH Study of HIV and Aging
- Principal Investigator(s): Monty Montano
- Study Contact: Brooke Ferguson Brawley; BBRAWLEY@PARTNERS.ORG; 617-525-9195
- Description of Subjects: Adult males and females, 50-65 years old. If HIV+, on effective ART.
- Location (Domestic vs. International): Boston Metropolitan Area
- Enrollment Period: 2015-2016
- Enrollment Goal: 200
- Key Exclusion and Inclusion Criteria: Must have a CD4 count > 350 cells/mm3 and HIV viral load < 200 copies/ml. Lower extremity mobility sufficient to participate in functional assessment
- Available Biological Specimens: Blood, serum, or plasma; Peripheral Blood Mononuclear Cells; Tissues and Stool; Other (Muscle tissue)
- How to Request Access: Contact Brooke Ferguson Brawley, BBRAWLEY@PARTNERS.ORG
- Available Data Description: multiple immune and muscle parameters
- Optional Additional Comments: This is a non-randomized observational longitudinal study design. We will recruit HIV infected adults (50 to 65 years old) and demographically matched uninfected control subjects to be followed for 4 years in this 5 year study. Total enrollment will be 200 subjects. The targeted age range of 50-65 is chosen to evaluate aging HIV infected individuals at risk for early frailty in the United States. Primary outcomes are measurement of a) inflammation, b) biomarkers for aging, c) CT scans of the mid-thigh, d) fibrosis in muscle and e) levels of physical function ability.
Pediatric HIV/AIDS Cohort Study (PHACS – multicenter 22 sites)
- Principal Investigator(s): Dr. Paige Williams & Dr. Sonia Hernandez-Diaz (HSPH) and Dr. Ellen Chadwick & Dr. Jennifer Jao (Northwestern)
- Study Contact: Liz Salomon, EdM, Program Director, 617-432-6762, lsalomon@hsph@harvard.edu
- Description of Subjects: Children, adolescents, and young adults living with perinatally acquired HIV (PHIV) or exposed to HIV at birth but uninfected (HEU), and their caregivers/birth mothers
- Location (Domestic vs. International): 21 clinical sites across the U.S., including Puerto Rico (see map of clinical sites)
- Enrollment Period: 2007-present
- Enrollment Goal: Enrollment is open and ongoing for all protocols.
- SMARTT: 5,000+ children with PHEU, along with their mothers/caregivers
- AMP Up Series: 700 young adults with PHIV and 250 young adults with PHEU
- TERBO BRAIN: 190 children ages 9-14 with PHEU, 100 young adults ages 22-29 (50 with PHIV, 50 with PHEU)
- Key Exclusion and Inclusion Criteria: Multiple protocols and sub-studies (see www.phacsstudy.org)
- Available Biological Specimens:
- Blood, serum, or plasma
- DNA
- Peripheral Blood Mononuclear Cells
- Tissues and Stool
- Exfoliated Teeth
- Hair Sample
- Lead Level
- Saliva Sample
- How to Request Access: See https://phacsstudy.org/.
- Available Data Description: All of the above, as well as cardiac echocardiograms, DEXA’s, extensive ND assessments
Pediatric HIV/AIDS Cohort Study (PHACS -BCH site only)
- Principal Investigator(s): Sandra Burchett (site leader), George Seage PI
- Study Contact: Sandra Burchett, MD, Tel. 617 355-6832, sandra.burchett@childrens.harvard.edu
- Description of Subjects: AMP:450 HIV+ youth, 150 HIV-controls; SMARRT: >1700 HIV neg/ARV exposed infants,youth. Study domains: cardiopulm, metabol, complications and HIV disease, maternal exposures, neuro, oral health, adolescent
- Location (Domestic vs. International): Domestic, Boston Children’s in MA but multicenter (George Seage, PI, HSPH)
- Enrollment Period: 2007-present
- Enrollment Goal: AMP:450 HIV+ youth, 150 HIV-controls; SMARRT: >1700 HIV neg/ARV exposed infants,youth.
- Key Exclusion and Inclusion Criteria: AMP: perinatal infection enroll between ages of 7-16, follow long term, database from birth. SMARRT: mother with HIV, infants exposed to ARV, uninfected, database from birth follow to 25
- Available Biological Specimens and Data: Yes, B-cell lines, Blood, serum, plasma, and DNA. Data from our Surveillance Monitoring for ART Toxicities (SMARTT) and Adolescent Master Protocol (AMP) studies are now available through NICHD DASH
- How to Request Access: Please contact Dr. Burchett with initial idea so she can appropriately link you with the proper working group.
- Mechanism for Requesting Samples: Database collaborations are welcome, and specimens may be shared. Multicenter, NICHD funded, AMP (HIV+/-youth); SMARRT(HIV-, ARV exposed babies.
- Available Data Description: CD4, VL, longitudinal data, co-infections, vaccines, neurodevelop testing, adherence, ARVs, acasi, oral health in HIV+, pulmonary ftn testing, echos, dexas, lab data
Phenogenetic HIV Negative
- Principal Investigator(s): Philip De Jager
- Study Contact: Laura Glick, 617-264-5947; lglick@partners.org or Tina Xue, txue@partners.org
- Description of Subjects: Self reported healthy, 18+, recallable for blood draw based on phenotype and genotype
- Location (Domestic vs. International): Domestic
- Enrollment Period: 2006 – ongoing
- Current Accrued Enrollment: 1753 current participants in the study
- Enrollment Goal: Planning to recruit 500 new participants every 5 years in order to sustain the subject population
- Key Exclusion and Inclusion Criteria: Inclusion: healthy individual, no history of autoimmune disorders, 18+
- Available Biological Specimens and Data: fresh blood samples, frozen serum, plasma, pbmc, dna for most
- How to Request Access: mailto:genestudy@partners.org
- Link to Cohort Website: http://dejager_lab.bwh.harvard.edu/?page_id=2317
Routine HIV testing in an Outpatient Department in Nakivale Refugee Settlement, Southwest Uganda
- Principal Investigator(s): Kelli O’Laughlin
- Study Contact: Kelli O’Laughlin, 617-525-9316, kolaughlin@partners.org
- Description of Subjects: refugees and Ugandan nationals attending an outpatient department health clinic in Nakivale Refugee Settlement in SW Uganda
- Location (Domestic vs. International): Nakivale Clinic in Nakivale Refugee Settlement in SW Uganda
- Enrollment Period: 2013-2014
- Enrollment Goal: 9,000
- Key Exclusion and Inclusion Criteria: Inclusion criteria: 1) 18 yrs or older, 2) able to provide informed consent, 3) not already known to be HIV positive
- Available Biological Specimens: none
- How to Request Access: email study contact directly kolaughlin@partners.org
- Available Data Description: rapid HIV test, CD4, linkage data, retention data, demographic survey, knowledge survey
Tshipidi: observational study of mortality and neurodevelopment among HIV-exposed/uninfected vs. HIV-unexposed infants in Botswana
- Principal Investigator(s): Shahin Lockman and Betsy Kammerer
- Study Contact: Shahin Lockman Tel: (617) 771-8780, slockman@hsph.harvard.edu
- Description of Subjects: 450 HIV-infected women and their babies, 450 HIV-uninfected women and their babies
- Location (Domestic vs. International): Gaborone and Mochudi, Botswana
- Enrollment Period: 2010-2012
- Enrollment Goal: 950 women and their infants (1900 total)
- Key Exclusion and Inclusion Criteria: Women are Botswana citizens >=18 years of age, able to provide informed consent
- Available Biological Specimens and Data: Blood, serum, or plasma, DNA, Peripheral Blood Mononuclear Cells, Other (breast milk (limited))
- How to Request Access: email study contact directly at slockman@hsph.harvard.edu
- Available Data Description: Longitudinal maternal/infant data for 2 years (including infant neurodevelopment); CD4, VL, heme/chem on subset