The HU CFAR is constantly leveraging new resources through program partners. We offer access to databases, repositories, specimens, clinical samples and information on research administration through our growing network of organizations across the HIV/AIDS research spectrum.
CNICS CFAR Network of Integrated Clinical Systems
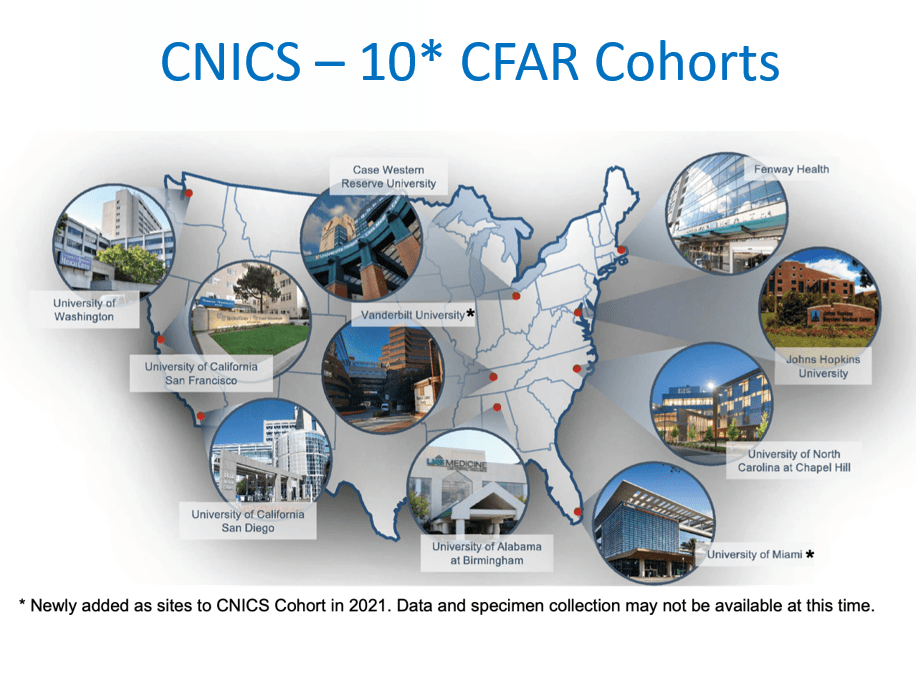
The CFAR Network of Integrated Clinical Systems (CNICS) offers access to the first electronic medical records-based resource network poised to integrate clinical data from the large and diverse population of HIV-infected persons in the modern HAART era.
CNICS supports HIV clinical outcomes and comparative effectiveness research. Data is collected from patients receiving care at one of eight US-funded Center for AIDS Research (CFAR) sites. CNICS captures a broad range of information associated with the rapidly changing course of HIV disease management.
CNICS addresses scientific questions that cannot be answered through other collaborative cohorts. CNICS collects comprehensive patient data including validated outcomes, longitudinal resistance data, and detailed patient reported outcomes (PROs) with readily available biological specimens.
Partners Infectious Disease Images
The Partners Infectious Disease Images website offers an extensive and easily searchable digital library of case information and images to facilitate learning infectious diseases and microbiology and to complement other teaching and learning formats. The site was developed with support from the National Library of Medicine by researchers at Partners HealthCare Inc., the University of Texas at Austin, Harvard Medical School, and Harvard Pilgrim Health Care, Inc., and with contributions from clinicians at Massachusetts General Hospital and Brigham and Women’s Hospital, national ID training programs through an affiliation with the Infectious Diseases Society of America, and other selected clinicians. The site is available free-of-charge. With support from the Harvard University Center for AIDS Research, Partners is expanding the digital library to include even more HIV- and TB-related cases, with a special interest in cases from international locations.
IDI Database Features:
- Digital library of case studies and images
- Special case collections focused on topics such as HIV/AIDS, Fungal Infections, Mycobacterial Infections and others
- References to abstracts and related articles on PubMed
- Cases with interactive annotations for medical students
- Browsing function for cases and images by organism, diagnosis, image technique, or lab technique
- Use of the site to perform self-assessment
- Optional creation of collections to save favorite cases and images
Research Compliance
HU CFAR Human Subjects and Vertebrate Animal Research Compliance:
If your HU CFAR-funded project involves human subjects, you must contact the office for human research protections at your institution, as well as at all sites involved in your study, to obtain IRB approval for your research. If your study protocol is covered by an approval for another study protocol, you will need to obtain an amended approval which must list the Harvard University Center for AIDS Research as a source of funds for your study, along with written verification that your protocol has been reviewed and is covered under the IRB approval.
If your HU CFAR-funded study involves the use of vertebrate animals, you must obtain and submit to the HU CFAR a signed IACUC approval letter and protocol. The approval will be vetted through the Harvard IACUC office by HU CFAR staff.
New Requirements Implement Broader Definition of Fetal Tissue:
For grant and cooperative agreement applications submitted for due dates on or after September 25, 2019, NIH is implementing new requirements (NOT-OD-19-128) on human fetal tissue (HFT) research for scientists applying for grants using human material from elective abortions.These new requirements apply to a far broader set of materials than what is conventionally considered HFT, to include extra-embryonic cells and tissue, such as umbilical cord tissue, cord blood, placenta, amniotic fluid, and chorionic villi, if obtained from the process of elective abortion as well as animal models incorporating HFT. Applications that do not address all of the required information will be administratively withdrawn and not reviewed by NIH. For more information, including what materials may be subject to them, read NIH’s FAQs and contact Melissa Lopes in Harvard’s OVPR. Read more…
Harvard University Research Compliance:
Resources are available through the Harvard Office of the Vice Provost for Research.
- Animal research and IACUCs
- Conflicts of interest and commitment
- Human subjects and IRBs
- Lab safety
- Research integrity
- Sponsored project administration
- Stem cells
- Access the new online module designed for Harvard researchers, “Financial Oversight of Sponsored Funding: What Researchers Need to Know.”
NIH Public Access Policy
The NIH Public Access Policy ensures that the public has access to the published results of NIH funded research.
Scientists are required to submit final peer-reviewed journal manuscripts that arise from NIH funds to the digital archive PubMed Central upon manuscript acceptance. Please read the tutorials available through Countway Library Services to learn how to submit manuscripts and review the Harvard University Guidelines around the NIH Public Access Policy. It is extremely important that all publications resulting from HU CFAR support comply with the NIH Public Access Policy. Failure to do so may result in federal withholding of HU CFAR funding. It is essential that all HU CFAR investigators understand the policy and submit publications for public access. If you have any questions or need assistance, please contact us.
View the latest NIH Public Access Policy Presentation.
There are two main requirements of the NIH Public Access Policy:
- Effective April 7, 2008 Principal Investigators must ensure that electronic versions of any peer-reviewed manuscripts arising from NIH funding and accepted for publication after that date are deposited in PubMed Central (PMC), NIH’s digital archive of biomedical and life sciences journal literature, and that the articles may be made publicly available by PMC no later than 12 months after publication.
- Effective May 25, 2008, anyone submitting an application, proposal, or progress report to NIH must include the PubMed Central reference number (PMCID) when citing articles that arise from their NIH funded research.
Manage Public Access and Your Professional Bibliography
eRA Commons has partnered with the National Center for Biotechnology Information (NCBI) to link NCBI’s personal online tool, “My NCBI,” to the Commons. My NCBI offers an online portal—“My Bibliography”—for users to maintain and manage a list of all of their authored works. As of April 2010, linking a Commons account to a new or existing My NCBI account allows references saved in My Bibliography to automatically appear in users’ Commons accounts. For more information about how to manage your professional bibliography in My NCBI, please follow the how-to links below.
NIH Bibliography Links:
My NCBI – Home:
http://www.ncbi.nlm.nih.gov/sites/myncbi/
Public Access Web site:
http://publicaccess.nih.gov
NIH Manuscript Submission System (NIHMS):
https://nihms.nih.gov
PubMed (pages contain link to My NCBI in top right corner):
http://www.ncbi.nlm.nih.gov/pubmed/
PubMed Central:
http://www.pubmedcentral.nih.gov
My NCBI: Managing Compliance with the NIH Public Access Policy Using My Bibliography (January 26, 2010):
http://www.nlm.nih.gov/pubs/techbull/jf10/jf10_myncbi_redesign.html
NIH Public Access Policy Compliance. : A Quick Guide for Harvard Researchers http://repository.countway.harvard.edu/hmscholar/HMscholarNIHGuide_FINAL.pdf
How to Manage your Bibliography Links:
- Get Started with My NCBI: Access My NCBI, Register, and Sign In
- Edit Your My Bibliography Settings (Add a Delegate)
- How to Log In to NIHMS Using MyNCBI Tutorial
- How to Submit a Manuscript in NIHMS Tutorial
My NCBI Frequently Asked Questions:
Monkeypox Resources
What You Need to Know:
- CDC is tracking an outbreak of monkeypox that has spread across several countries that don’t normally report monkeypox, including the United States.
- The monkeypox virus is spreading mostly through close, intimate contact with someone who has monkeypox.
- You can take steps to prevent getting monkeypox and lower your risk during sex.
- CDC recommends vaccination for people who have been exposed to monkeypox and people who are at higher risk of being exposed to monkeypox.
- If you have any symptoms of monkeypox, talk to your healthcare provider, even if you don’t think you had contact with someone who has monkeypox.
- CDC is urging healthcare providers in the United States to be alert for patients who have rash illnesses consistent with monkeypox.
Federal Resources: Monkeypox and HIV:
- Clinicalinfo.HIV.gov — Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV, Monkeypox Update
- HRSA Ryan White HIV/AIDS Program — Monkeypox Resources
Substance Use Scientific Working Group (SU SWG) Consultation Service
This free consultation service will provide guidance and assistance on biobehavioral and clinical trial design involving substance use disorders and HIV treatment and prevention, substance use assessment, as well as drug testing and basic science related to substance use. Drs. Abigail Batchelder and Peter Chai, HU CFAR Substance Use Scientific Working Group (SU SWG) directors, will field requests and identify members of the SU SWG with relevant expertise to provide the consultation.
Adolescence and HIV Toolkit
Click here to access the toolkit resources.

Search Biostatistics Resources
Search HIV Cohorts
The HU CFAR Clinical Core connects HIV-related research cohorts with resources. In turn, many of these studies have data and specimens available for further investigation. Use the categories on the right to browse the available cohorts.
If your research can benefit from access to data and specimens from these Cohorts, please reach out to the respective study contact.
If you wish to list a study in the HU CFAR Cohort Catalogue, please submit it directly via the Cohort Submission Form.
RESEARCH COHORTS (View all Cohorts available here)
